Starting from February next year: Shanghai will review and approve the evaluation and approval of Class II medical devices
On December 23, 2016, the Shanghai Food and Drug Administration issued a notice on optimizing the approval process for the second-class medical device review in this Municipality, and the first registration, renewal registration, licensing change, and registration of the second type of equipment in the jurisdiction. The review and approval process for matters such as changes will be optimized and will be implemented on February 1, 2017.
Taking a closer look, the Shanghai Bureau's optimization measures are really awesome: data merger acceptance, review approval time limit compression, exemption registration quality system verification... In order to let everyone understand more intuitively, Xiaobian integrates optimization measures and steps into the registration flow chart. ,For reference:
- First registration -
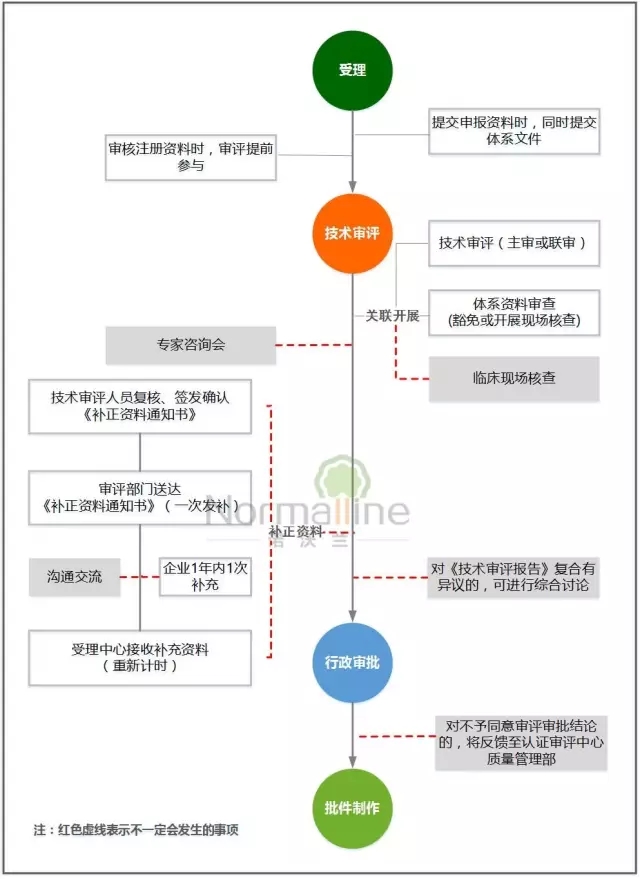
Double synchronization: that is, when receiving the registration application, the corresponding quality management system data can be received synchronously, reducing the round-trip of the enterprise and improving the efficiency of circulation; the technical review and registration quality system verification, and the clinical trial site verification are carried out simultaneously to improve work coordination;
Double advance: the technical reviewers intervene in the acceptance process in advance, improve the quality of the accepted data, and ensure that the registration fee is smooth and orderly; the issuer of the technical review is involved in the review process in advance, which facilitates the unified information correction and effective control Time limit is assessed to avoid secondary replenishment.
frozen red spot swimming crab
Frozen Crab,Delicious Frozen Crab,Frozen Red Spot Swimming Crab,Fresh Frozen Red Swimming Crab
Zhoushan Junwei Aquatic Products Co., Ltd. , https://www.junweiaquatic-intl.com